Recently, the research group of Professor Haoshen Zhou and Associate Professor Shaohua Guo reported a new type of lithium-rich rock-salt oxide, which is based on the charge compensation mechanism of cation and anion. It has a very stable discharge capacity at a large rate and almost no voltage decay. The related work was titled A New Type of Li-Rich Rock-Salt Oxide Li2Ni1/3Ru2/3O3 with Reversible Anionic Redox Chemistry, published online in Advanced Materials on January 23, 2019 (Adv. Mater. 2019, 201807825 ).
The layered lithium-rich material has more active lithium ions that can participate in the deintercalation behavior during charge and discharge, and provides a specific capacity greater than 250 mAh g-1 compared to positive electrode materials (such as lithium cobalt oxide, lithium iron phosphate, etc.). Besides the transition metal valence mechanism, the mechanism of oxygen activation has also been discovered during the charging and discharging process. The activation of oxygen provides the possibility of large-capacity behavior of the material. However, oxygen activation induces irreversible oxygen deficiency that changes the layered structure to spinel phase, leading to a capacity and voltage decay of layered lithium-rich materials during cycling. The research group of Haoshen Zhou and Shaohua Guo developed a new type of Fd-3m cubic lithium-rich rock-salt oxide Li2Ni1/3Ru2/3O3, which has a stable structure, large capacity, outstanding rate capability as well as good cycling performance with negligible voltage decay. The research group characterized the structural stability of the material by in-situ X-ray diffraction, confirmed the oxygen activation behavior by in-situ Raman and in-situ gas mass spectrometry, and supported material stability at a high rate by first-principles calculations.

Figure 1. Electrochemical performances of Li2Ni1/3Ru2/3O3 as the positive electrodes. a) Typical initial two charge-discharge profiles between 2.0 and 4.3 V with the current density of 10 mA g−1. b) Cycling performance of the electrode at a high current density of 200 mA g−1 . c) The charge/discharge profiles with normalized capacity for the first 150 cycles at the current density of 200 mA g−1 . d) GITT results of Li2Ni1/3Ru2/3O3 for the first cycle with the variation of quasi-equilibrium potentials and the calculated Li+ diffusion coefficient.
The capacity retention rate is above 94%after 150 cycles at a current density of 200 mA g-1. As shown in Figure 1c, the curves of the first circle and the 150th circle basically overlap, and the voltage decay is unnoticeable, revealing the outstanding stability of the rock-salt material. The theoretical calculation also indicates a high diffusion coefficient of lithium ions at high rate, owning to the new Fd-3m rock-salt structure. Raman spectroscopy can be used as a fingerprint spectrum to determine covalent bonds between substances. In the Raman spectrum at the early stage of charging, no peroxygen bond is generated. At the later stage of charging, the peroxygen bond appears and subsequently blue shifts, indicating that the bond length is getting shorter and reaches the shortest at the end of the charge. During the discharge process, the peroxygen bond exhibits symmetry behaviors to the charging process, as same as in the second cycle, indicating the reversibility of the peroxygen bond, and the ability of oxygen brings extra-large capacity through reversible price changing. Superoxide appears for a second at the end of the first circle but disappears in the second circle, suggesting the stability peroxygen bonds and indicating there is no relation between the formation of superoxide and peroxygen bonds. This material is promising in the future design of large-capacity cathode materials because of its advantages in the stability of the capacity and discharge potential.
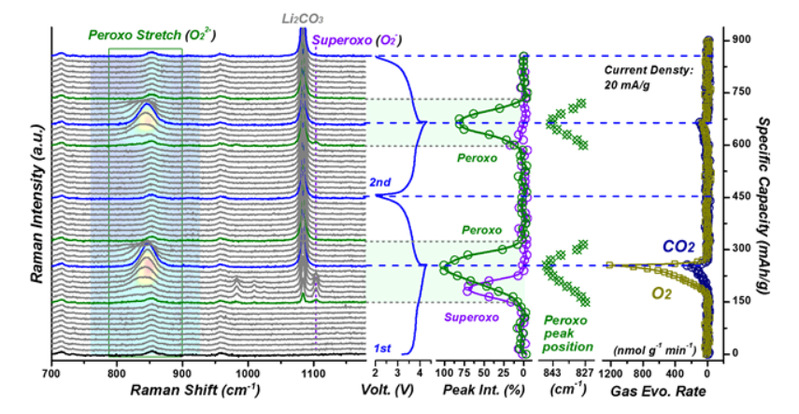
Figure 2. The results of in situ Raman spectra for the initial two galvanostatic cycles (20 mA g−1)
Graduate candidate Xiang Li and Dr. Yu Qiao contributed equally to this work. This work was partially supported financially by National Basic Research Program of China (2014CB932300), NSF of China (21633003, 51802149, 11704245, and 11874199) and NSF of Jiangsu Province, China (BK20170630).

