Rechargeable aqueous Zn–MnO2 batteries (AZMBs) have emerged as a compelling energy storage solution due to their outstanding cost-effectiveness, inherent safety, and high theoretical capacity, making them particularly suitable for grid and off-grid energy systems. However, the intrinsic properties of the Zn and MnO2 electrodes (e.g., structural stability, electrochemical activity, and diffusion kinetics) as well as the composition and stability of the electrolyte (e.g., solvation behavior in the Helmholtz layer and the solvation structure of hydrated Zn2+ ions) impose significant limitations. These challenges necessitate further optimization of the stability and compatibility of the electrode–electrolyte interfaces (EEIs) during operation. Specifically, the Zn anode–electrolyte interface (AEI) is plagued by persistent issues such as the hydrogen evolution reaction (HER), Zn corrosion, and dendrite formation. Simultaneously, the MnO2 cathode-electrolyte interface (CEI) faces challenges such as substantial volume expansion, sluggish diffusion kinetics, and undesirable side reactions, including Mn dissolution, proton insertion, and parasitic electrolyte decomposition, which collectively degrade the cycling stability and electrochemical performance. These challenges collectively undermine the reversibility and cycle life of AZMBs, posing significant obstacles to their practical implementation.
Recently,professor Yagang Yao’s Group at Nanjing universityintroduces a simple and scalable engineering strategy to establish a stable bilayer interface between electrodes and electrolytes by incorporating sodium thioctate (ST) molecules into ZnSO4 electrolytes. The introduction of zincophilic ST molecules first optimizes the electrolyte environment by modulating the solvation structure of hydrated Zn2+ ions, partially displacing associated H2O molecules from the solvation sheath, and thereby mitigating corrosion while suppressing HER. Subsequently, the specific adsorption of electronegative ST anions at the inner Helmholtz plane (IHP) reconstructs the EDL structure, simultaneously promoting the desolvation of hydrated Zn2+ ions and homogenizing charge distribution via Zn2+-capturing capability. Upon adsorption, the in-situ polymerization of interfacial ST molecules constructs a bilayer architecture comprising a ZnS–organic amorphous inner layer and a poly(zinc thioctate) (PZT) outer layer, where the former regulates Zn2+ ion flux while the latter enhances mechanical robustness. Remarkably, the dynamic cleavage and recombination of disulfide bonds in ST molecules endow the polymeric interface with self-healing properties, enabling damage repair and further enhancing the self-regulating and adaptive capabilities of the EEI. As a result of these unique features, ST-enhanced Zn symmetric cells achieve an ultralong cycle life of 7,800 cycles at a high current density of 60 mA cm−2. Furthermore, the assembled AZMBs demonstrate exceptional cycling stability, with a negligible capacity decay rate of 0.0014% over 10,000 cycles at 2,000 mA g−1. This bilayer interface engineering strategy represents a significant advance in accelerating the development of long-lifespan AZMBs.
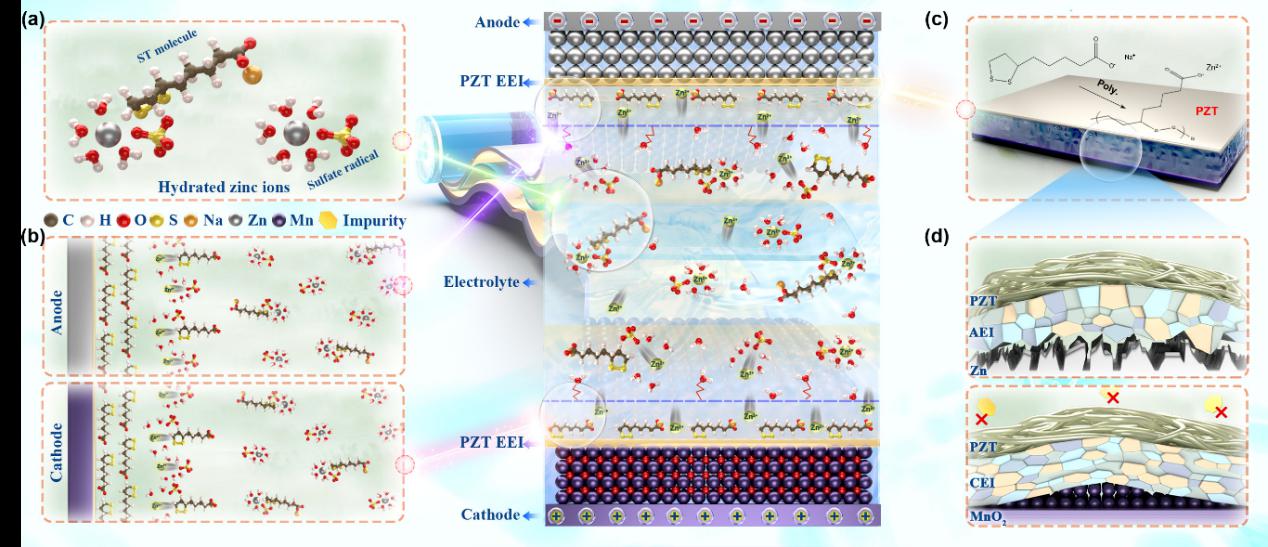
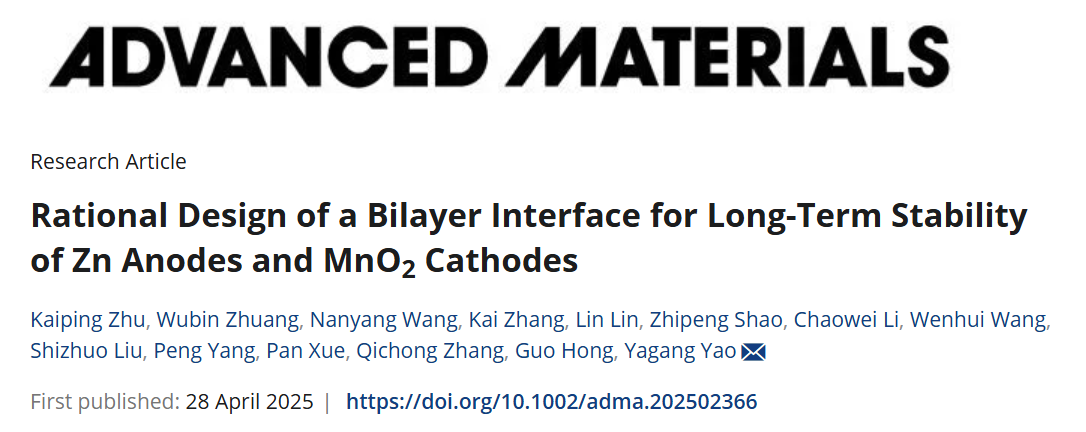
Figure 1 illustrates the formation mechanism and potential advantages of the bilayer interface. In this work, an electrolyte additive strategy was employed to optimize the solvation structure of the electrolyte, resulting in the construction of a bilayer structure consisting of a ZnS–organic amorphous inner layer and a PZT outer layer.
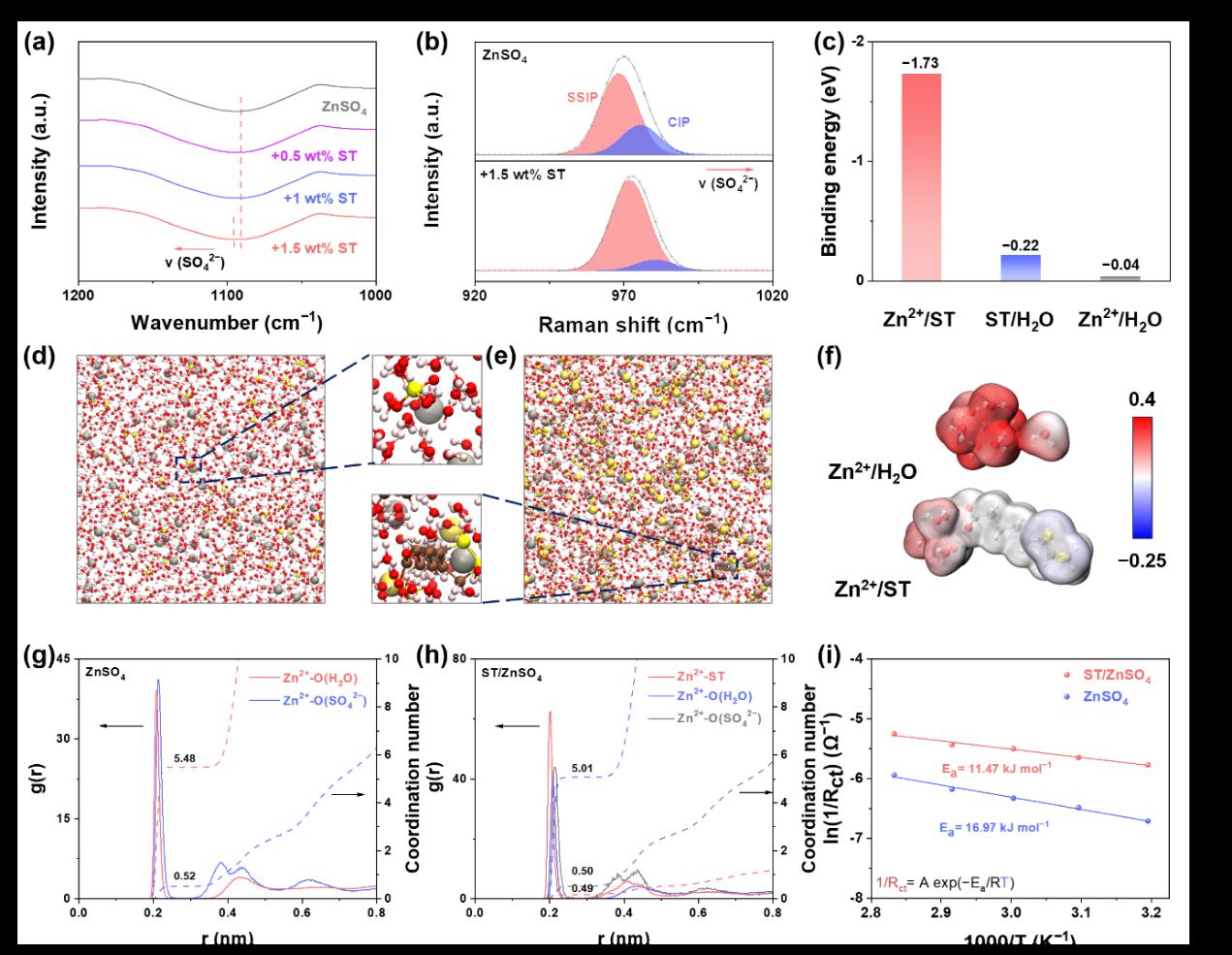
Figure 2 illustrates the functional mechanism of ST molecules in the electrolyte. The introduction of ST molecules optimizes the solvation structure of the electrolyte, effectively suppressing zinc corrosion and the hydrogen evolution side reaction.
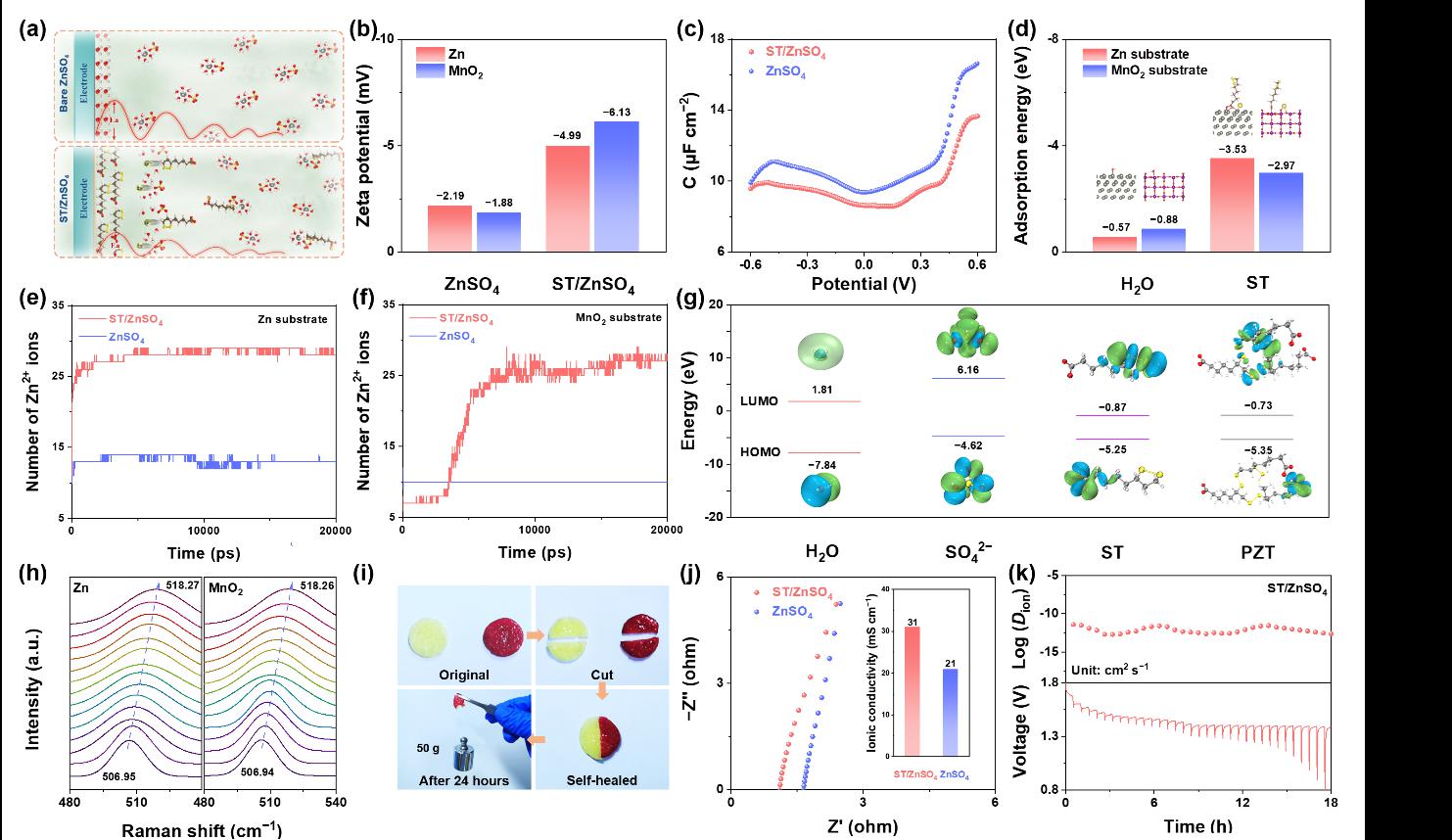
Figure 3 illustrates the formation mechanism of ST molecules at the EEI.The specific adsorption of electronegative ST anions at the inner Helmholtz plane (IHP) reconstructs the EDL structure, simultaneously promoting the desolvation of hydrated Zn2+ ions and homogenizing charge distribution via Zn2+-capturing capability. Upon adsorption, the in-situ polymerization of interfacial ST molecules constructs a bilayer architecture comprising a ZnS–organic amorphous inner layer and a poly(zinc thioctate) (PZT) outer layer, where the former regulates Zn2+ ion flux while the latter enhances mechanical robustness. Remarkably, the dynamic cleavage and recombination of disulfide bonds in ST molecules endow the polymeric interface with self-healing properties, enabling damage repair and further enhancing the self-regulating and adaptive capabilities of the EEI.
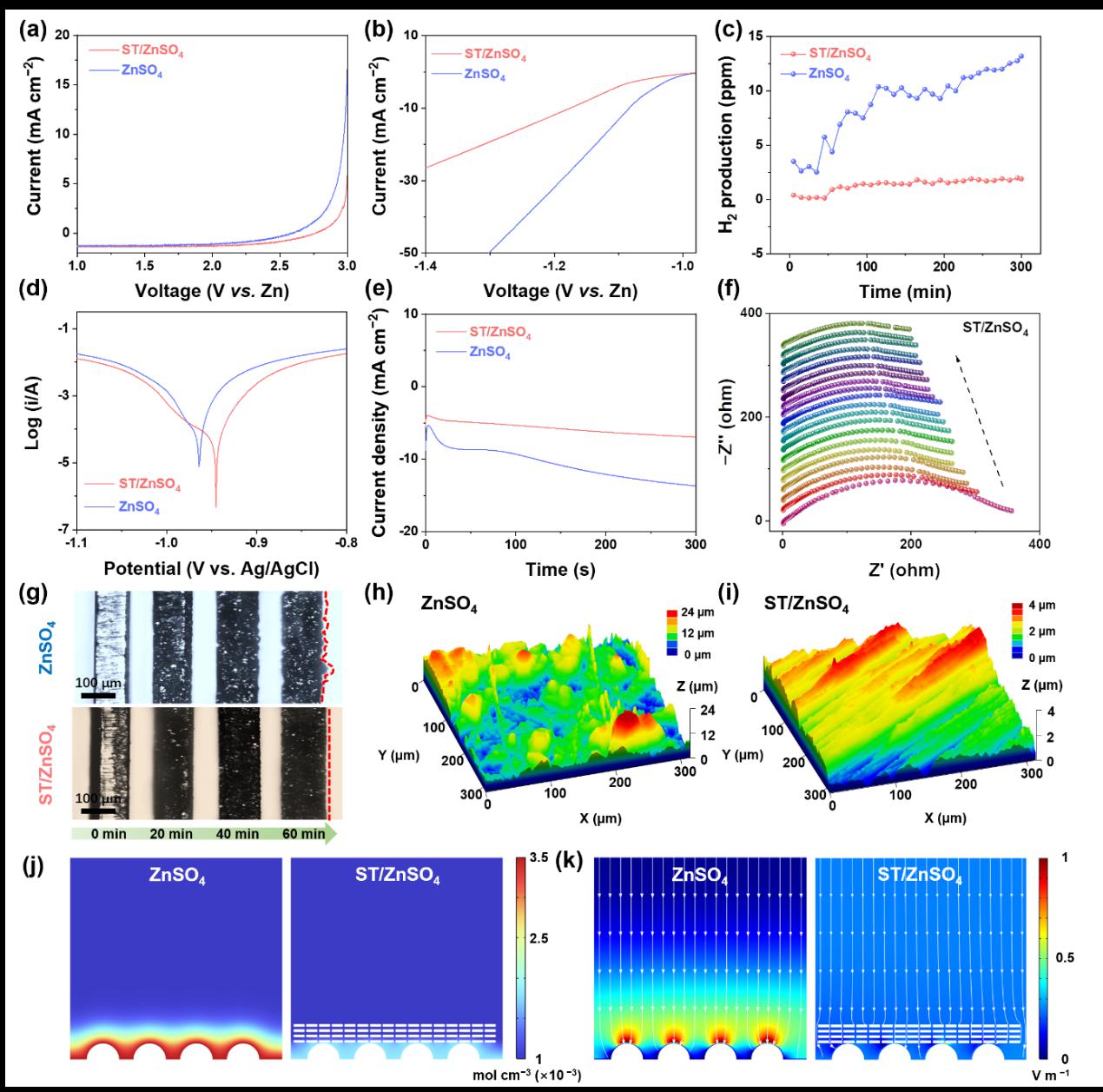
Figure 4 demonstrates that ST molecules effectively suppress zinc corrosion and hydrogen evolution side reactions, promote the formation of a stable electrode interface, and induce uniform zinc deposition, resulting in a smooth and homogeneous zinc morphology, thereby extending the service life of the zinc anode.
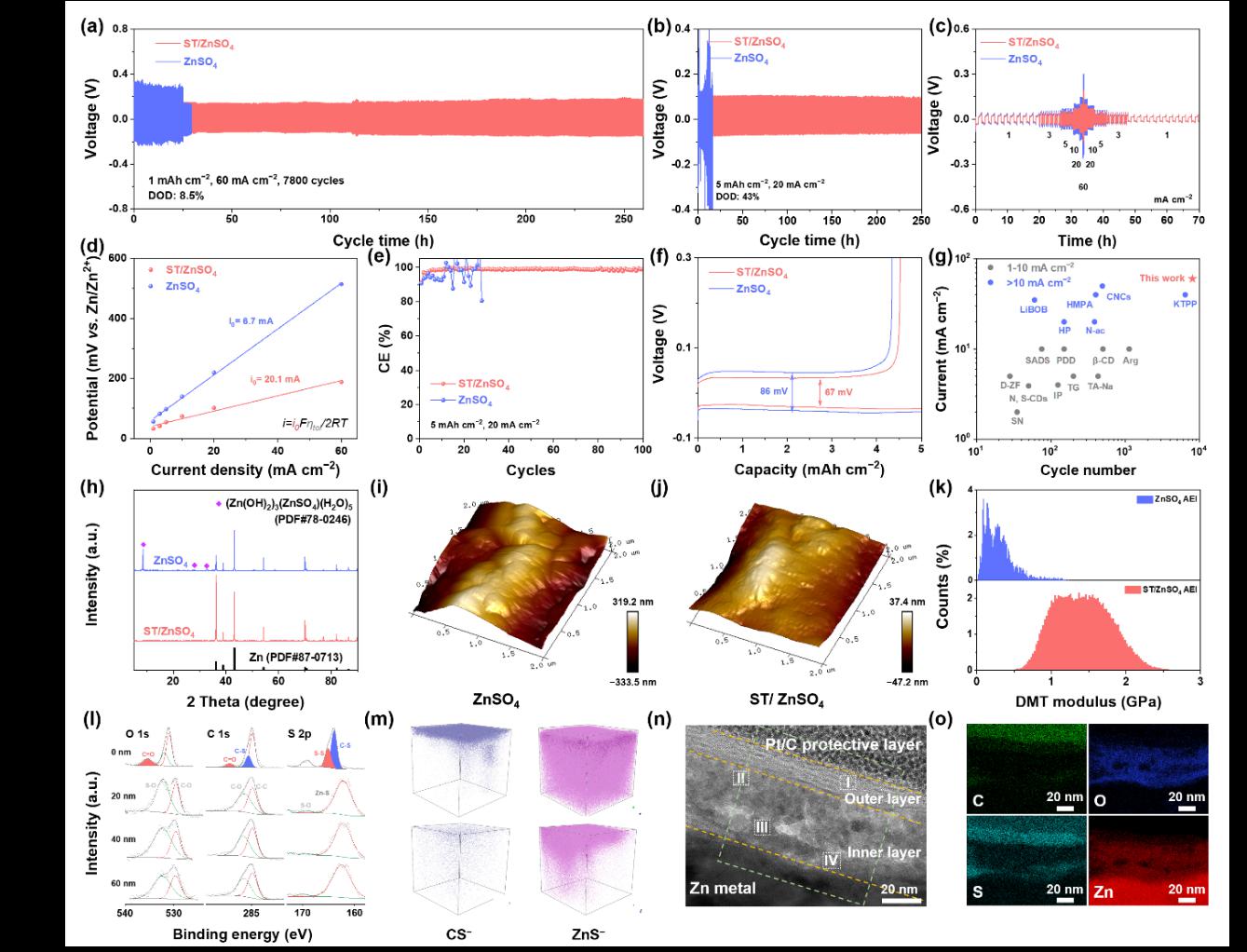
Figure 5 presents the performance and formation mechanism of ST molecules at the interface between the zinc anode and the electrolyte. The formation of this bilayer structure significantly enhances the mechanical strength and chemical stability of the Zn anode–electrolyte interface, effectively preventing direct contact between H₂O in the electrolyte and the zinc electrode, thereby suppressing side reactions. Owing to this unique design, the ST-enhanced zinc symmetric cell achieves an ultra-long cycle life of up to 7,800 cycles under a high current density of 60 mA cm⁻².

Figure 6 presents the performance and formation mechanism of ST molecules at the interface between the MnO₂ cathode and the electrolyte. The constructed bilayer cathode–electrolyte interface effectively suppresses interfacial side reactions, enhances interfacial stability, and improves electrochemical performance. With this unique design, the assembled Zn–MnO₂ full battery exhibits an extremely low capacity decay rate of only 0.0014% after 10,000 cycles at 2000 mA g⁻¹. This bilayer interface engineering strategy represents a significant breakthrough toward the development of long-lifespan Zn–MnO₂ full batteries.
The related research work, titled “Rational Design of a Bilayer Interface for Long-Term Stability of Zn Anodes and MnO₂ Cathodes,” was published in Advanced Materials, a top-tier journal in the field of materials science. Kaiping Zhu, a Ph.D. candidate, and Wubin Zhuang, a master's student from the College of Engineering and Applied Sciences at Nanjing University, are the co-first authors of the paper. This work was supported by the National Key R&D Program of China (2024YFE0109200), the Fundamental Research Funds for the Central Universities (No. 2024300440), and Guangdong Basic and Applied Basic Research Foundation (2025A1515011098). The work also received strong support and assistance from the National Laboratory of Solid State Microstructures, the College of Engineering and Applied Sciences, the Jiangsu Key Laboratory of Artificial Functional Materials, the Collaborative Innovation Center of Advanced Microstructures at Nanjing University, and the Shenzhen Research Institute of Nanjing University.
Article link:https://doi.org/10.1002/adma.202502366

